|
Study - Results of Double Blind
Preliminary Results of Multi-Centered, Double Blind, Placebo Controlled, Cross-Over Study Evaluating Endogenous hGH (Human Growth Hormone) Levels with Serial hGH (Human Growth Hormone) Radio -Immunoassay Levels After Trans-Dermal GH Releasing Hormone Analog (Trans-D Tropin®) Administration.Rashid Buttar, DO, James Biddle, MD, Rajiv Chandra, MD, Terry Grossman, MD,Clarence Norris, MD, James Smith, DO, Annette Stoesser, MD, Dean Viktora, PhD Serial hGH (Human Growth Hormone) radio-immunoassay testing has clearly established rapid and substantial increases in ENDOGENOUS hGH (Human Growth Hormone) levels with Tran-D Tropin® usage. This tran -dermal GH Releasing Hormone analog offers the first hope of naturally and conveniently sustaining youthful levels of hGH (Human Growth Hormone). The only definitive method for precise evaluation of GH treatment is by DIRECT MEASUREMENT of ENDOGENOUS hGH (Human Growth Hormone). However, this test usually is not obtained by the clinician. One reason for this is, up until now, no generally available GH therapeutic modality has ever been shown to effectively increase ENDOGENOUS hGH (Human Growth Hormone) levels in a sustainable manner. As a result, the testing of hGH (Human Growth Hormone) has usually been reserved for evaluation in hGH (Human Growth Hormone) deficiency and short stature syndromes. Another major reason why hGH (Human Growth Hormone) levels have not been measured as a standard is because natural physiological release of ENDOG ENOUS hGH (Human Growth Hormone) is pulsatile. Therefore, the very transitory nature of ENDOGENOUS hGH (Human Growth Hormone) makes it difficult to measure. The preliminary results of a double blind study demonstrated measurably increased levels of ENDOGENOUS hGH (Human Growth Hormone) per radio-immunoassay in 117 patients using Trans-D Tropin®. Serum hGH (Human Growth Hormone) levels were drawn at baseline, followed by a dose of Trans-D Tropin® (experimental group) or placebo (control group) with subsequent serum levels drawn at 30, 60 and 90 minutes post treatment. Average levels increased o ver 750 within 30 minutes of Trans-D Tropin® application. ENDOGENOUS hGH (Human Growth Hormone) levels increased 462% from baseline to 90 minutes after Trans-D Tropin® administration during first time use. At 2 weeks, over 815% increase in ENDOGENOUS hGH (Human Growth Hormone) level were recorded from baseline to 90 minutes post Trans-D Tropin® application. By week 5, a 1754 increase in ENDOGENOUS hGH (Human Growth Hormone) levels were achieved within 90 minutes of using Trans-D Tropin®, compared to baseline. Although every patient did not respond (93.16% response rate), the data was statistically significant (P<0.001). In addition to increasing ENDOGENOUS hGH (Human Growth Hormone) levels, Trans-D Tropin® demonstrated change not generally associated with hGH (Human Growth Hormone) injection therapy. Consistent decrease in Cortisol, Insulin and IGF-1 levels were noted. The majority of published medical literature and current research have definitively established the unreliability of IGF-1 as an indicator of hGH (Human Growth Hormone) therapy efficacy. This study further indicates an actual inverse relationship between IGF-1 and hGH (Human Growth Hormone) treatment. Rapid and dramatic improvements in muscle strength, endurance, insomnia, anorexia, and sense of well being were also noted. Placebo group showed same subjective changes when crossed over into experimental group. Tran -D Tropin® appear to be not only more efficacious, but the safety, convenience, cost advantage, compliance and natural physiological emulation are factors which make it a far more preferable treatment modality for increasing hGH (Human Growth Hormone) levels than recombinant, synthetic hGH (Human Growth Hormone) injection therapy. % Change in Endogenous hGH (Human Growth Hormone) by Radio -Immunoassay in 117 Patients (Change from Baseline to 90 Minutes Over 8 Week Period, Using Trans-D Tropin®) Drop at 8 wks in hGH (Human Growth Hormone) attributed to either Somatostatin stimulation or drop in pituitary reserves due to sustained GH release. Subjective responses (SF-36 patient outcome based) improved beyond 8 wks. At 18 months, improvements continue. Evidence: Subjective response i intact, despite minimal hGH (Human Growth Hormone) change 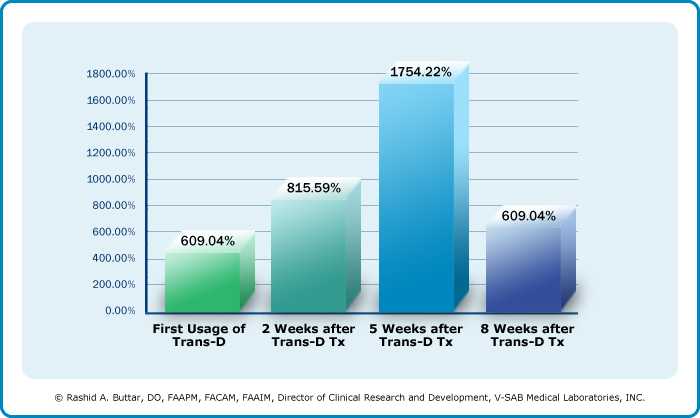
|
|
These statements have not been evaluated by the US Food and Drug Administration (FDA). This information is not intended to diagnose, treat, cure or prevent any disease. Terms of Use | Privacy Statement ©2008-2020 Trans-D Tropin All Rights Reserved. |










